We perform tests of face masks in accordance with EN 14683
We perform all required tests of medical masks in accordance with the harmonized standard EN 14683.
EU type-examination is the part of the conformity assessment procedure in which the notified body examines the technical design of the PPE, and verifies and attests that the technical design of the PPE meets the requirements of the Regulations that apply to it.
Provided the initial assessment procedure is successfully completed, a certificate of approval of the internal production control system and controlled testing of products at random intervals shall be issued. This is valid for three years from the beginning of the assessment process, if all the conditions satisfy the requirements of the Regulation (EU) 2016/425.
If the initial assessment is successfully completed, the certificate of approval of the quality system is issued. It is valid for three years from the beginning of the assessment process, if all the conditions satisfy the requirements of the Regulation (EU) 2016/425.
We are one of only 5 laboratories in the EU which can offer such a wide range of services.
All tests are performed in brand new laboratories equipped with modern testing equipment.
The reason we have such a great case study here
is that we are professional and flexible.
And we are able to deliver results
in a very short period of time.
About the integrity of an individual
who has signed the papers.
To either sell or buy or use
a piece of standardized equipment
such as the face masks.
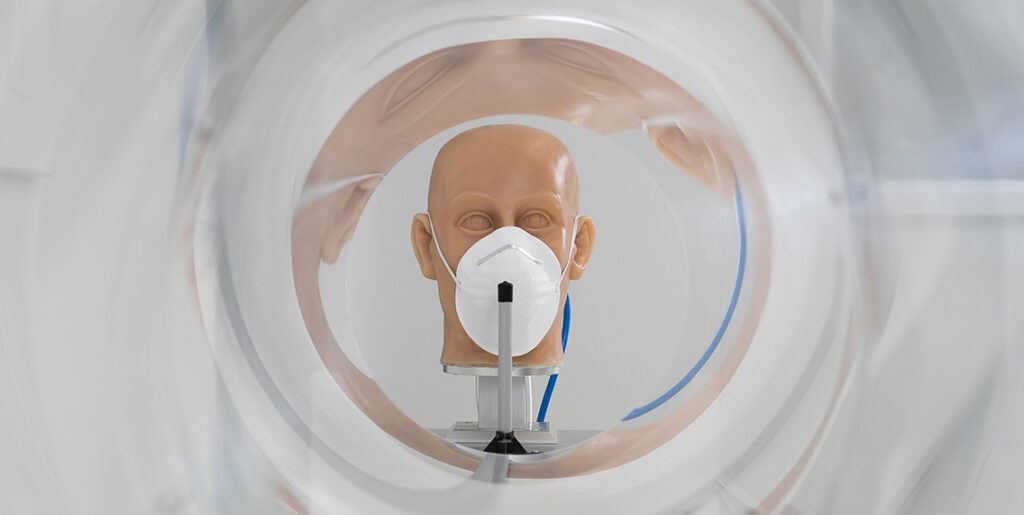

We perform all required tests of medical masks in accordance with the harmonized standard EN 14683.
Choosing the right mask is a daunting task.
For the purpose of expert review of the documentation of medical or protective masks, we in LOTRIČ Metrology prepare a Technical Report with detailed descriptions of all established facts.